Room-temperature NaI/H2O compression icing: solute–solute interactions
Abstract
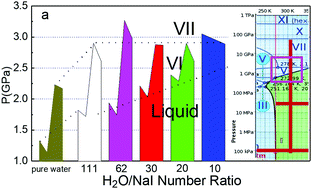
In situ Raman spectroscopy revealed that transiting the concentrated NaI/H2O solutions to an ice VI phase and then into an ice VII phase at 298 K proceeds in a way different from that activated by the solute type. Unlike the solute type that raises both the critical pressures PC1 and PC2, for the liquid–VI, the VI–VII transition simultaneously occurs in the Hofmeister series order: I > Br > Cl > F ∼ 0; concentration increase raises the PC1 faster than the PC2 that remains almost constant at higher NaI/H2O molecular number ratios. Concentration increase moves the PC1 along the liquid–VI phase boundary and it finally merges with PC2 at the triple-phase junction featured at 350 K and 3.05 GPa. The highly-deformed H–O bond is less sensitive to the concentration because of the involvement of anion–anion repulsion that weakens the electric field in the hydration shells. Observations confirm that the salt solvation lengthens the O:H nonbond and softens its phonon but relaxes the H–O bond contrastingly. Compression, however, has the opposite effect from that of salt solvation. Therefore, compression recovers the polarization-deformed O:H–O bond first and then proceeds to the phase transitions. The anion–anion interaction discriminates the effect of NaI/H2O concentration from that of the solute type at an identical concentration on the phase transitions.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...