Adsorption of molecular hydrogen on coronene with a new potential energy surface
Abstract
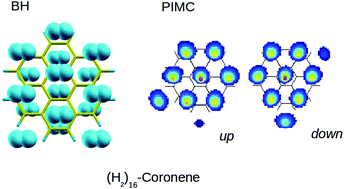
Benchmark interaction energies between coronene, C24H12, and molecular hydrogen, H2, have been computed by means of high level electronic structure calculations. Binding energies, equilibrium distances and strengths of the long range attraction, evaluated for the basic configurations of the H2–C24H12 complex, indicate that the system is not too affected by the relative orientations of the diatom, suggesting that its behavior can be approximated to that of a pseudoatom. The obtained energy profiles have confirmed the noncovalent nature of the bonding and serve to tune-up the parameters of a new force field based on the atom-bond approach which correctly describes the main features of the H2–coronene interaction. The structure and binding energies of (para-H2)N–coronene clusters have been investigated with an additive model for the above mentioned interactions and exploiting basin-hopping and path integral Monte Carlo calculations for N = 1–16 at T = 2 K. Differences with respect to the prototypical (rare gas)N–coronene aggregates have been discussed.


 Please wait while we load your content...
Please wait while we load your content...