Indirect NMR detection of transient guanosyl radical protonation in neutral aqueous solution†
Abstract
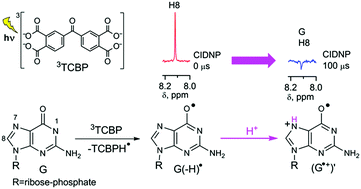
By using the time-resolved chemically induced dynamic nuclear polarization technique, we show that the neutral guanosyl radical, G(-H)˙, formed in the reaction of guanosine-5′-monophosphate with a triplet-excited 3,3′,4,4′-tetracarboxy benzophenone in neutral aqueous solution, protonates readily at the N7 position with the formation of a new guanosyl cation radical (G˙+)′.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...