The promotion effects of thionation and isomerization on charge carrier mobility in naphthalene diimide crystals
Abstract
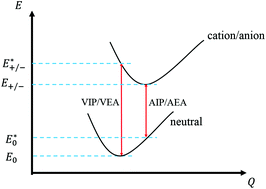
Herein, the promotion effects of thionation and isomerization on the carrier mobility properties of naphthalene diimide and thionated naphthalene diimide crystals were investigated in detail based on the Marcus–Hush theory and quantum-chemical calculations. The thionation of NDIs will improve the charge mobility of both electrons and holes, which is similar to the thionation of PDIs. The compound P only behaves as an n-type organic semiconductor (OSC), whereas the three other thionation structures have higher mobility values and can behave as p-type OSCs. For the cis/trans isomers of the two double-thionation structures, trans-S2 has a larger hole and electron carrier mobility than cis-S2; this is consistent with the experimental results obtained for cis–trans-isomers. A potential strategy for the development of high performance ambipolar OSCs is the substitution of O atoms by S atoms. These results will provide a guide for the design and optimization of OSCs via analysis of the relationship between carrier mobility and molecular crystal structures.


 Please wait while we load your content...
Please wait while we load your content...