The pressure–temperature phase diagram of pure Co based on first-principles calculations
Abstract
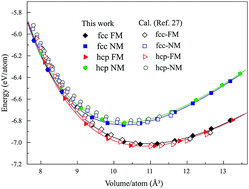
We optimized the high pressure–temperature phase diagram of pure Co up to the liquidus temperature and 120 GPa, based on thermodynamic properties calculated using first-principles. The Gibbs energy for each phase was evaluated in the framework of a quasiharmonic approximation, with a consideration of the thermal electronic contribution at finite temperatures. Particularly, the liquidus temperature, as a function of pressure, was determined using classical Molecular Dynamics simulations. Our results in this work successfully integrated experimental observations and the previous theoretical predictions. The critical solid phase transitions of ε → γf and ε → β were clarified using the Gibbs energy as a function of pressure and temperature. In addition, the magnetism of β above 70 GPa was verified to be nonmagnetic. The difference between γp and β, which was unclear before, has been illustrated to be associated with the magnetic transformation from the paramagnetic state of the γp phase to the nonmagnetic state of the β phase rather than the structural transformation.


 Please wait while we load your content...
Please wait while we load your content...