The role of van der Waals and exchange interactions in high-pressure solid hydrogen
Abstract
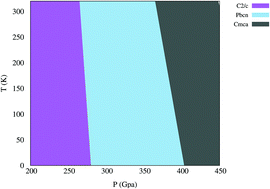
We investigate the van der Waals interactions in solid molecular hydrogen structures. We calculate enthalpy and the Gibbs free energy to obtain zero and finite temperature phase diagrams, respectively. We employ density functional theory (DFT) to calculate the electronic structure and density functional perturbation theory (DFPT) with van der Waals (vdW) functionals to obtain phonon spectra. We focus on the solid molecular C2/c, Cmca-12, P63/m, Cmca, and Pbcn structures within the pressure range of 200 < P < 450 GPa. We propose two structures of the C2/c and Pbcn for phase III which are stabilized within different pressure range above 200 GPa. We find that vdW functionals have a big effect on vibrations and finite-temperature phase stability, however, different vdW functionals have different effects. We conclude that, in addition to the vdW interaction, a correct treatment of the high charge gradient limit is essential. We show that the dependence of molecular bond-lengths on exchange–correlation also has a considerable influence on the calculated metallization pressure, introducing errors of up to 100 GPa.


 Please wait while we load your content...
Please wait while we load your content...