Origin of attraction in p-benzoquinone complexes with benzene and p-hydroquinone†
Abstract
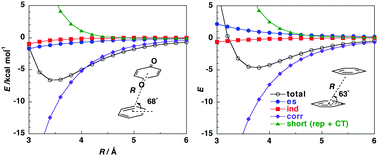
The origin of the attraction in charge-transfer complexes (a p-hydroquinone–p-benzoquinone complex and benzene complexes with benzoquinone, tetracyanoethylene and Br2) was analyzed using distributed multipole analysis and symmetry-adapted perturbation theory. Both methods show that the dispersion interactions are the primary source of the attraction in these charge-transfer complexes followed by the electrostatic interactions. The natures of the intermolecular interactions in these complexes are close to the π/π interactions of neutral aromatic molecules. The electrostatic interactions play important roles in determining the magnitude of the attraction. The contribution of charge-transfer interactions to the attraction is not large compared with the dispersion interactions in these complexes.


 Please wait while we load your content...
Please wait while we load your content...