Ionic diffusion and proton transfer in aqueous solutions of alkali metal salts
Abstract
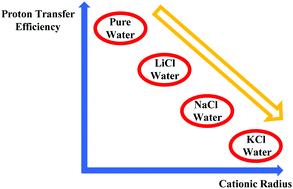
We report on a series of ab initio molecular dynamics investigations on LiCl, NaCl, and KCl aqueous solutions under the effect of static electric fields. We have found that although in low-to-moderate field intensity regimes the well-known sequence of cationic mobilities μ(K+) > μ(Na+) > μ(Li+) (i.e., the bigger the cation the higher the mobility) is recovered, from intense field strengths this intuitive rule is no longer verified. In fact, field-induced water molecular dissociations lead to more complex phenomena regulating the standard migration properties of the simplest monovalent cations. The water dissociation threshold is lowered from 0.35 V Å−1 to 0.25 V Å−1 by the presence of charged species in all samples. However, notwithstanding a one-stage process of water ionization and proton conduction takes place at 0.25 V Å−1 in the electrolyte solutions where “structure maker” cations are present (i.e., LiCl and NaCl), the KCl aqueous solution shows some hindrance in establishing a proton conductive regime, which is characterized by the same proton conduction threshold of neat water (i.e., 0.35 V Å−1). In addition, it turns out that protons flow easily in the LiCl (σp = 3.0 S cm−1) solution and then – in descending order – in the NaCl (σp = 2.5 S cm−1) and KCl (σp = 2.3 S cm−1) electrolyte solutions. The protonic conduction efficiency is thus inversely proportional to the ionic radii of the cations present in the samples. Moreover, Cl− anions act as a sort of “protonic well” for high field intensities, further lowering the overall proton transfer efficiency of the aqueous solutions. As a consequence, all the recorded protonic conductivities are lower than that for neat water (σp = 7.8 S cm−1), which strongly indicates that devices exploiting the proton transfer ability should be designed so as to minimize the presence of ionic impurities.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...