Folic acid supramolecular ionogels
Abstract
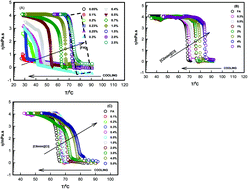
Herein, we report on folic acid (FA, a low molecular weight gelator) thermoreversible supramolecular organo (in 1 : 1 (v/v) water–DMSO binary solvent), and ionogels made in 1-ethyl-3-methyl imidazolium chloride, [C2mim][Cl], and 1-octyl-3-methyl imidazolium chloride, [C8mim][Cl], solutions with 0.1 ≤ [IL] ≤ 5% (w/v). The self-assembled fibrils of folic acid were largely formed due to secondary forces, such as π–π stacking, H-bonding, and hydrophobic interactions above a gelator concentration of 0.2% (w/v) at room temperature, 20 °C. Fragile gels (FGs) having a low frequency storage modulus G0 ≈ 4–6 Pa were formed when 0.2 ≤ [FA] ≤ 0.5% (w/v) in the binary solvent, and at higher gelator concentration (0.5 ≤ [FA] ≤ 2.5% (w/v)) formation of strong gels (SGs) with a G0 value of 140 Pa–5 kPa was noticed. In the IL environment, for a given gelator concentration of [FA] = 1% (w/v), SG formation (with G0 ≈ 2–5 kPa) was noticed when 0.1 ≤ [IL] ≤ 0.5% (w/v), whereas very strong gels (VSGs) with remarkably high gel strengths were formed with G0 ≈ 11–15 kPa. Gelation temperature Tgel could be varied from 45 to 75 °C by varying the FA concentration in the binary solvent, whereas the ionogels exhibited an almost 10 °C rise in gelation temperature. The information obtained from the relative network density νr (ratio of network density in iono- to organo gel), differential free-energy and enthalpy of gelation, ΔGW–IL and ΔHW–IL (difference in free-energy and enthalpy of organo to ionogel), implied that [C2mim][Cl] ionogels had enhanced homogeneity, and higher crosslink density and gel strength. A 3-D plot of Tgel and G0versus gelator concentration clearly defines a phase diagram that describes the contour of the gelation domains of this biologically important gelator.


 Please wait while we load your content...
Please wait while we load your content...