Perturbation of cationic equilibrium by cucurbit-7-uril†
Abstract
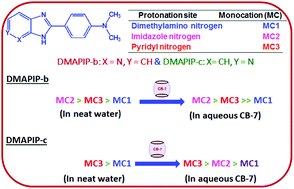
The effect of cucurbit-7-uril (CB-7) on a cationic mixture with same charge has been investigated by studying monocationic mixtures of 2-(4′-N,N-dimethylaminophenyl)imidazo[4,5-b]pyridine (DMAPIP-b) and 2-(4′-N,N-dimethylaminophenyl)imidazo[4,5-c]pyridine (DMAPIP-c). The pKa of both the guests increases in CB-7. DMAPIP-b forms all three monocations in the ground and the excited states in aqueous as well as in CB-7 solution. However, CB-7 shifts the equilibrium more towards the less polar MC2 and MC3. On the other hand, DMAPIP-c exists only as MC1 and MC3 in aqueous solution, however, in CB-7 it exists not only as MC1 and MC3 but also as MC2 in CB-7 in the ground state. In the excited state, as in aqueous solution MC1 forms MC2 by biprotonic phototautomerism in CB-7. The association constants of monocations suggest a pyridyl nitrogen position dependence. All the MC–CB-7 complexes are optimized by density functional theory (DFT).

 Please wait while we load your content...
Please wait while we load your content...