Trends of intramolecular hydrogen bonding in substituted alcohols: a deeper investigation†
Abstract
Intramolecular hydrogen bonding (IAHB) is one of the most important intramolecular interactions and a critical element in adopted molecular arrangements. Moreover, slight substitution in a molecule can affect its strength to a great extent. It is well established that alkyl groups play a positive role in IAHB strength. However, the effects that drive it are specific to each system. To investigate the influence of IAHB and its strength dependency on different acyclic compounds, the conformational preferences of propane-1,3-diol, 3-methoxypropan-1-ol, 3-ethoxypropan-1-ol, 3-isopropoxypropan-1-ol, 3-(tert-butoxy)propan-1-ol, butane-1,3-diol, 3-methoxybutan-1-ol, 3-methylbutane-1-diol, and 3-methoxy-3-methylbutan-1-ol were evaluated experimentally using infrared spectroscopy theoretically supported by topological and natural bond orbital analyses. The most stable conformation of each compound exhibited IAHB and these conformers are more populated in the equilibrium for all studied compounds. Experimental infrared and topological data suggest that the strength of IAHB increases for each methyl group addition. NBO analyses indicate that methyl groups in different positions related to an OH moiety affect the charge transfer energy involved in intramolecular hydrogen bonding. This occurs mostly due to an increase in the spn-hybridized lone pair (LP1O) contribution to the charge transfer 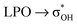 , which is a result of changes in s-character and orbital energy caused by geometrical rearrangements, rehybridization, and/or electronic effects.
, which is a result of changes in s-character and orbital energy caused by geometrical rearrangements, rehybridization, and/or electronic effects.
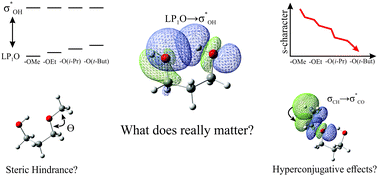


 Please wait while we load your content...
Please wait while we load your content...