Approaching complexity of alkyl hydrogenation on Pd via density-functional modelling†
Abstract
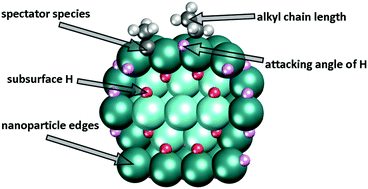
Pd is widely used to catalyse hydrogenation and dehydrogenation reactions. One of them is the hydrogenation of ethylene, which includes the transformation of ethyl species to ethane. Herein, by means of density-functional calculations we address several still insufficiently understood factors affecting the latter process. In particular, we shed light on the following aspects of hydrogenation of alkyls on Pd: (i) the mechanistic details of how subsurface H accelerates the reaction on a (111) surface; (ii) the role of nanoparticle edges; and (iii) the influence of a common spectator ethylidyne, ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–CH3. These factors are identified as significant for the height of the ethyl hydrogenation barrier on Pd. Moreover, we show that butyl hydrogenation on Pd is also governed by very similar interactions, which suggests a broader applicability of our conclusions. This study highlights the complexity of alkyl hydrogenation and analyses the factors that need to be taken into account for a more realistic description of the hydrogenation processes on metal surfaces.
C–CH3. These factors are identified as significant for the height of the ethyl hydrogenation barrier on Pd. Moreover, we show that butyl hydrogenation on Pd is also governed by very similar interactions, which suggests a broader applicability of our conclusions. This study highlights the complexity of alkyl hydrogenation and analyses the factors that need to be taken into account for a more realistic description of the hydrogenation processes on metal surfaces.


 Please wait while we load your content...
Please wait while we load your content...