Unravelling hydrogen bonding interactions of tryptamine–water dimer from neutral to cation†
Abstract
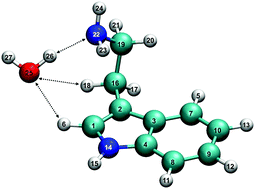
The neutral and cationic forms of tryptamine–water dimer present a variety of noncovalent interactions. To characterize these interactions, a series of complementary methods, including the quantum theory of atoms in molecules, noncovalent interaction plots, natural bond orbital analysis, and energy decomposition analysis, were used. For the first time, the existence of the three intermolecular H-bonds in the conformer-locked tryptamine–water dimer A–H2O are identified, highlighting a single water's role as one proton donor and two proton acceptors as it binds to tryptamine. Furthermore, upon threshold ionization of the A–H2O dimer, the network of the three intermolecular H-bonds is indeed preserved while the individual H-bonds’ binding strengths are subject to change; this is attributed to the existence of the optically accessible minimum energy isomer A+–H2O in the cation. In addition, it is found that the global minimum energy isomer H+–H2O contains a single intermolecular H-bond, but is more stable, by ca. 3 kcal mol−1, than the local minimum energy isomer A+–H2O; this is due to the stronger intramolecular interaction of H+–H2O as opposed to A+–H2O.


 Please wait while we load your content...
Please wait while we load your content...