Nucleophilicity and electrophilicity of the C(sp3)–H bond: methane and ethane binary complexes with iodine†
Abstract
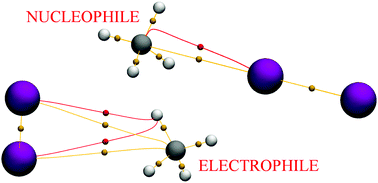
The occurrence of stable van der Waals complexes of small saturated hydrocarbons with molecular iodine is assessed in order to investigate the ability of sp3-hybridized carbon atoms to act as either electron donors or electron acceptors depending on the ligand orientation. Systematic ab initio potential energy surface exploration of methane–I2 and ethane–I2 model dimers was followed by thorough characterization. Despite modest evidence of whole-adduct polarization, the resulting interactions feature a dominant dispersive character. The noncovalent interaction descriptors employed comprise NBO, AIM, NCI, and source function analyses. The relevance of bonding C–H orbitals in donor–acceptor interactions involving saturated hydrocarbons is highlighted. The results here presented corroborate existing literature regarding the electrophilicity of the aliphatic C–H group, and also indicate that the nucleophilic character of C(sp3) shares a dependence on electron withdrawing/donating substituents similar to that extensively documented for σ-holes. Indeed, the sole difference between the two, aside from the nucleophilicity/electrophilicity switch, seems to lie in their directionality. Nucleophilic sites on C(sp3) are not limited to the outermost region of C along a covalent bond axis, but can also engage electrophiles via the bifurcation plane of a CH2 unit. Since valence electrons on these carbon atoms are engaged in covalent bonds, they can only interact with polarizing ligands via the electron density accumulation/depletion in the four corresponding σ orbitals. These, however, do not seem to interact individually with the accompanying electrophile. Source function and NCI results suggest instead that nucleophilic carbon centres participate in the noncovalent bond themselves by drawing electron density from their shared electron pairs.


 Please wait while we load your content...
Please wait while we load your content...