Periodic density functional theory analysis of direct methane conversion into ethylene and aromatic hydrocarbons catalyzed by Mo4C2/ZSM-5†
Abstract
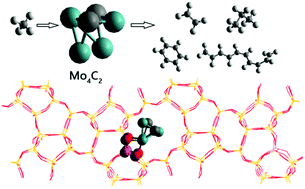
Mo/ZSM-5-catalyzed methane conversion into aromatic hydrocarbons is an important reaction to produce ethylene and benzene, but the detailed reaction mechanism has not been investigated due to its high complexity. In the present study, density functional theory combined with a periodic model was used to investigate the reaction mechanism of direct methane conversion into aromatic hydrocarbons catalyzed by Mo/ZSM-5. The calculation results show that the active phase for Mo is Mo4C2 instead of MoOx. The whole reaction processes processed via the following steps: the C–H bond in methane was first activated by Mo4C2 with an energy barrier of 1.01 eV and then converted into ethylene species via the coupling of two CH3 species as well as two successive dehydrogenation steps (2CH3 → C2H6 → C2H4 + 2H). The rate-controlling step for the processes to form ethylene is the coupling of two methyl species with a barrier of 1.22 eV. The produced ethylene species then react with each other to produce C6H8via the reaction of 3C2H4 → C3H8 + 2H2, and molecular benzene is formed by successive dehydrogenation of C6H8. The rate-limiting step for benzene formation from ethylene is the cyclization step of chain C6H8 with an energy barrier of 1.21 eV. Additionally, molecular propane (C3H8) is formed by the reaction of C2H4 + CH4 → C3H8, and the controlling step C3H7 + H → C3H8 has a barrier of 1.46 eV. Molecular C10H12 is produced via coupling of C6H8 and C2H4, where the limiting step is the dehydrogenation step of C8H12 with an energy barrier of 1.44 eV. Our present calculation results indicate that the selectivity of benzene was the largest among the possible products, that is, C2H4, C3H6, C6H6 and C10H12, based on the corresponding controlling step barrier. Importantly, the rate-controlling step for the whole reaction process from methane to benzene is the dissociative adsorption of methane (CH4 → CH3 + H) with an energy barrier of 1.83 eV when considering entropy contribution. The present study may help people design a good catalyst for the formation of benzene from methane; in other words, the catalyst should have a good ability to activate the C–H bond of molecular methane.


 Please wait while we load your content...
Please wait while we load your content...