Ionic liquids containing tricyanomethanide anions: physicochemical characterisation and performance as electrochemical double-layer capacitor electrolytes†
Abstract
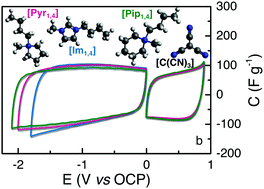
We investigated the use of fluorine free ionic liquids (ILs) containing the tricyanomethanide anion ([C(CN)3]) as an electrolyte in electrochemical double-layer capacitors (EDLCs). Three cations were used; 1-butyl-3-methylimidazolium ([Im1,4]), N-butyl-N-methylpyrrolidinium ([Pyr1,4]) and N-butyl-N-methylpiperidinium ([Pip1,4]). Their physicochemical properties are discussed alongside with their performance as electrolytes. We found that the cyano-based ILs present higher ionic conductivity (9.4, 8.7 and 4.2 mS cm−1 at 25 °C for [Im1,4], [Pyr1,4] and [Pip1,4], respectively) than the widely studied IL containing the bis(trifluoromethylsulfonyl)imide anion, namely [Pyr1,4][Tf2N] (2.7 mS cm−1 at 25 °C). Of the three ILs investigated, [Pip1,4][C(CN)3] presents the widest electrochemical stability window, 3.0 V, while [Pyr1,4][C(CN)3] is stable up to 2.9 V and its [Tf2N] analogue can operate at 3.5 V. Despite operating at a lower voltage, [Pyr1,4][C(CN)3] EDLC is capable of delivering up to 4.5 W h kg−1 when operating at high specific power of 7.2 kW kg−1, while its [Pyr1,4][Tf2N] counterpart only delivered 3.0 W h kg−1 when operated at similar power.
- This article is part of the themed collection: SBQ-RSC: Celebrating UK-Brazil collaborations


 Please wait while we load your content...
Please wait while we load your content...