A quantitative measure of halogen bond activation in cocrystallization†
Abstract
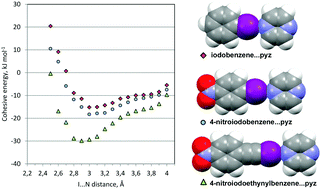
A theoretical investigation of bond lengths and bond energies for several kinds of halogen bonding interactions is carried out using the PIXEL method. The effect of different kinds of activating agents, fluoro-, nitro-, ethynyl substitution and combinations thereof, is assessed quantitatively, and is found to be fully consistent with the results of literature screenings of the corresponding strengths, as judged by the ease of formation of cocrystals. In the best combination of activators the halogen bond is comparable or superior to a strong O–H⋯O hydrogen bond in what concerns stabilization energies and stretching force constants. At least with iodine acceptors, in our picture the halogen-bonding effect is a localized interaction arising from the detail of the electron distribution at the halogen atom, mainly of a Coulombic-polarization nature but with dispersion energies contributing significantly. Binding energies correlate with the electrostatic potential at the tip of the halogen and even with Mulliken population analysis atomic charges, providing easily accessible guidelines for crystal engineers. For one typical cocrystal structure the analysis of separate molecule–molecule energies reveals the nature of the packing forces and rank halogen bonding as the main influence, closely followed by coplanar stacking of coformers.


 Please wait while we load your content...
Please wait while we load your content...