Effect of mixed anions on the physicochemical properties of a sodium containing alkoxyammonium ionic liquid electrolyte†
Abstract
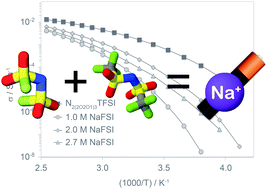
The physicochemical properties of the ionic liquid N-ethyl-2-(2-methoxyethoxy)-N,N-bis(2-(2-methoxyethoxy)ethyl)ethan-1-ammonium bis(trifluoromethylsulfonyl)imide (N2(2O2O1)3TFSI) as well as its solutions with sodium bis(trifluoromethylsulfonyl)imide (NaTFSI) and sodium bis(fluorosulfonyl)imide (NaFSI) are compared in order to study the effects of the anion. The NaFSI solutions show weaker interactions as suggested by lower glass transition temperatures, lower densities, lower viscosities and higher conductivities as compared to their more strongly coordinating NaTFSI analogues. The transport properties follow Vogel–Tamman–Fulcher behaviour suggesting that the mixtures are fragile glass formers. The addition of a higher concentration of Na salts results in decoupling of the translational motion from the viscosity, as observed from Walden plots, and 23Na NMR data suggests that the sodium speciation is independent of the nature of the Na salt or the temperature but is affected by the salt concentration.


 Please wait while we load your content...
Please wait while we load your content...