Mechanism of ultrafast non-reactive deactivation of the retinal chromophore in non-polar solvents†
Abstract
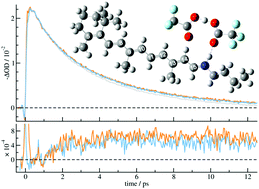
The photoisomerization of the all-trans protonated Schiff base of retinal (SBR+) in solution is highly inefficient. The present theoretical and experimental investigation aims at disclosing the mechanisms of ultrafast, non-reactive relaxation of SBR+ that lead to the drastic decrease in the isomerization yield in non-polar solvents. Our pump–probe measurements demonstrate the sensitivity of the all-trans SBR+ excited-state dynamics on the electrostatic interaction with the surrounding counterions and the crucial importance of the chromophore microenvironment. Our computational study focuses for the first time on the retinal chromophore–counterion pairs that are formed in non-polar solvents. By employing TDDFT-based nonadiabatic dynamics simulations and ADC(2) reaction paths calculations we found that internal conversion from the initially excited state to an inter-molecular charge transfer state with excitation localized on the counterion, leads to dissociation of the chromophore–counterion pair and to the abortion of isomerization. Barriers to conical intersection with the inter-molecular charge transfer state were found in the range 0.42–0.67 eV at the ADC(2) level. The existence of a barrier along the non-reactive relaxation pathways explains the observation that in solution the excitation on the blue edge of the SBR+ absorption leads to decrease in the isomerization yield with respect to the excitation at the red edge.
- This article is part of the themed collection: XUV/X-ray light and fast ions for ultrafast chemistry


 Please wait while we load your content...
Please wait while we load your content...