The reaction of Criegee intermediate CH2OO with water dimer: primary products and atmospheric impact†
Abstract
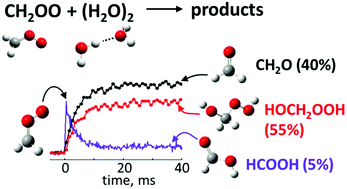
The rapid reaction of the smallest Criegee intermediate, CH2OO, with water dimers is the dominant removal mechanism for CH2OO in the Earth's atmosphere, but its products are not well understood. This reaction was recently suggested as a significant source of the most abundant tropospheric organic acid, formic acid (HCOOH), which is consistently underpredicted by atmospheric models. However, using time-resolved measurements of reaction kinetics by UV absorption and product analysis by photoionization mass spectrometry, we show that the primary products of this reaction are formaldehyde and hydroxymethyl hydroperoxide (HMHP), with direct HCOOH yields of less than 10%. Incorporating our results into a global chemistry-transport model further reduces HCOOH levels by 10–90%, relative to previous modeling assumptions, which indicates that the reaction CH2OO + water dimer by itself cannot resolve the discrepancy between the measured and predicted HCOOH levels.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...