Intricate kinetics: in situ FTIR-spectroscopy discloses a phase change during ionic liquid synthesis†
Abstract
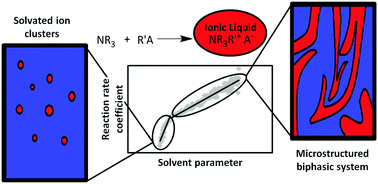
The kinetics of the methylation of triethylamine (NEt3) with dimethyl carbonate (DMC) in methanol have been investigated by means of in situ ATR-IR-spectroscopy. The data show an autocatalytic influence of the ionic product on reaction kinetics. A bend in the reaction rate progress indicates a sudden change from solvated ions to a microstructured biphasic system, which is reflected in the dependence of the reaction rate constant on the product concentration and the Hildebrand parameter.


 Please wait while we load your content...
Please wait while we load your content...