The water dimer reaction OH + (H2O)2 → (H2O)–OH + H2O
Abstract
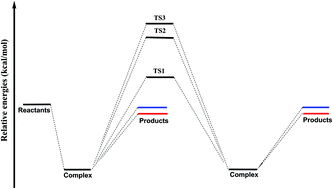
The stationary points, including the entrance complex, transition states, and the exit complex, for the reaction OH + (H2O)2 → (H2O)OH + H2O have been carefully examined using the “gold standard” CCSD(T) method with the correlation-consistent basis sets up to cc-pVQZ. The complex (H2O)2⋯OH is found to lie 10.8 kcal mol−1 below the separated reactants. This complex should be observable in the gas phase via vibrational or microwave spectroscopy. Seven unique transition states were found. One pathway for the title reaction has no barrier, in which the OH radical captures a whole water molecule from the water dimer. For the hydrogen abstraction pathways the lowest classical barrier height is predicted to be 5.9 kcal mol−1 (TS1) relative to separated reactants, and the other pathways are of higher barriers, i.e., 17.8 (TS2) and 18.4 (TS3) kcal mol−1. The harmonic vibrational frequencies and the zero-point vibrational energies of the stationary points for the reaction are also reported.


 Please wait while we load your content...
Please wait while we load your content...