Methanol oxidation on stoichiometric and oxygen-rich RuO2(110)
Abstract
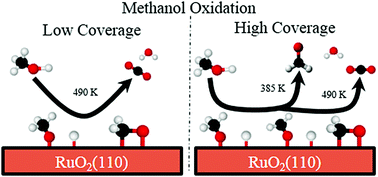
We used temperature-programmed reaction spectroscopy (TPRS) to investigate the adsorption and oxidation of methanol on stoichiometric and O-rich RuO2(110) surfaces. We find that the complete oxidation of CH3OH is strongly preferred on stoichiometric RuO2(110) during TPRS for initial CH3OH coverages below ∼0.33 ML (monolayer), and that partial oxidation to mainly CH2O becomes increasingly favored with increasing CH3OH coverage from 0.33 to 1.0 ML. We present evidence that an adsorbed CH2O2 species serves as the key intermediate to complete oxidation and that CH2O2 formation is intrinsically facile but becomes limited by the availability of bridging O-atoms on stoichiometric RuO2(110) at initial CH3OH coverages above 0.33 ML. We show that methanol molecules adsorbed in excess of 0.33 ML dehydrogenate to mainly CH2O and desorb during TPRS, with adsorbed CH3O groups mediating the evolution of both CH2O and CH3OH. We find that O-rich RuO2(110) surfaces are also highly active toward methanol oxidation and that selectivity toward the complete oxidation of methanol increases markedly with increasing coverage of on-top O-atoms (Oot) on RuO2(110). Our results demonstrate that CH3OH species adsorbed within Oot-rich domains react efficiently during TPRS, in parallel with reaction of CH3OH adsorbed initially on cus-Ru sites. The data suggests that the facile hydrogenation of Oot atoms and the resulting desorption of H2O at low-temperature (<∼400 K) provides an efficient pathway for restoring reactive O-atoms and thereby promoting complete oxidation of methanol on the O-rich RuO2(110) surface.


 Please wait while we load your content...
Please wait while we load your content...