Kinetic differences in the intercalation of linear and cyclic penta(ethylene oxide)s into graphite oxide leading to separation by topology†
Abstract
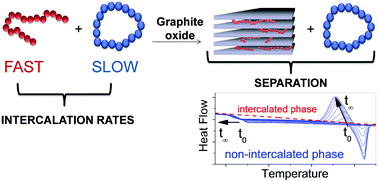
In the present study, the structural differences between linear and cyclic oligo(ethylene oxide)s are demonstrated to cause large kinetic differences in the intercalation of both topologies into the interlayer space of graphite oxide (GO). This study is performed with room-temperature liquid oligomers: penta(ethylene glycol), penta(ethylene glycol) dimethyl ether, 15-crown-5 and 2-hydroxymethyl-15-crown-5. Cyclic compounds exhibit 100 to 1000 times slower intercalation rates than the linear oligomers and exhibit induction periods of several hours prior to intercalation. This enormous difference in the intercalation rate resulted in the selective exclusion of cyclic molecules from the linear ones in experiments performed with their blends, as evidenced by the evolution of glass transition and crystallization. An increase in the concentration of the cyclic molecules in the non-intercalated liquid from 70 wt% to values as high as 99 wt% was accomplished. This study reveals the potential use of selective intercalation mediated by GO as a tool for separation of cyclic and linear oligo(ethylene oxide)s.


 Please wait while we load your content...
Please wait while we load your content...