Balanced work function as a driver for facile hydrogen evolution reaction – comprehension and experimental assessment of interfacial catalytic descriptor†
Abstract
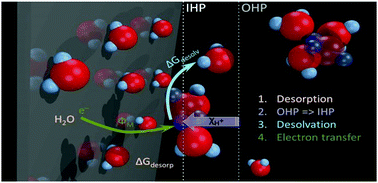
A major step in the development of (electro)catalysis would be the possibility to estimate accurately the energetics of adsorption processes related to reaction intermediates. Computational chemistry (e.g. using DFT) developed significantly in that direction and allowed the fast prediction of (electro)catalytic activity trends and improved the general understanding of adsorption at electrochemical interfaces. However, building a reliable and comprehensive picture of electrocatalytic reactions undoubtedly requires experimental assessment of adsorption energies. In this way, the results obtained by computational chemistry can be complemented or challenged, which often is a necessary pathway to further advance the understanding of electrochemical interfaces. In this work an interfacial descriptor of the electrocatalytic activity for hydrogen evolution reaction, analogue to the adsorption energy of the Had intermediate, is identified experimentally using in situ probing of the surface potentials of the metals, under conditions of continuous control of the humidity and the gas exposure. The derived activity trends give clear indication that the electrocatalytic activity for hydrogen evolution reaction is a consequence of an interplay between metal–hydrogen and metal–water interactions. In other words it is shown that the M–H bond formation strongly depends on the nature of the metal–water interaction. In fact, it seems that water dipoles at the metal/electrolyte interface play a critical role for electron and proton transfer in the double layer.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...