Positron annihilation characteristics, water uptake and proton conductivity of composite Nafion membranes
Abstract
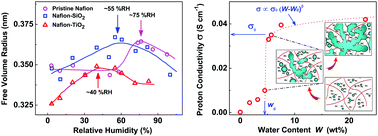
The free volumes and proton conductivities of Nafion membranes were investigated at different humidities by positron annihilation lifetime spectroscopy (PALS) and using an electrochemical workstation, respectively. The results showed that the variation in o-Ps lifetime τo-Ps was closely associated with the microstructure evolution and the development of hydrophilic ion clusters in Nafion membranes as a function of water uptake, regardless of metal oxide additives. In particular, with increasing relative humidity, the maximum value of τo-Ps in the Nafion membranes corresponded to the formation of numerous water channels for proton transportation. Numerous well-connected water channels in Nafion–TiO2 hybrid membranes could be formed at a much lower relative humidity (∼40% RH) than in the pristine one (∼75% RH), due to the better water retention ability of the Nafion–TiO2 membranes. Further, a percolation behavior of proton conductivity at high water uptake in Nafion membranes was observed, which showed that the percolation of ionic-water clusters occurred at the water uptake of ∼4.5 wt%, and ∼6 wt% was basically enough for the formation of a well-connected water channel network.


 Please wait while we load your content...
Please wait while we load your content...