CO diffusion as a re-orientation mechanism in the NaY zeolite†
Abstract
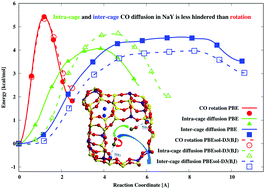
Our work is devoted to DFT calculations of the relative rotational and diffusional barriers for CO motions in zeolite NaY. The diffusion jump of CO adsorbed in NaY from NaII to Na′II has been confirmed as the favored way for CO re-coordination via either the C or the O atom to the Na cations instead of the CO rotation, hence explaining the mechanism which is responsible for the CO exchange between different positions and the changes in the intensities of the vibrational IR spectra. The fine structure of the vibrational C–O bands is explained by the different CO locations of adsorbed mono- and dicarbonyl species. The calculated activation energy of intra-cage CO diffusion from NaII–CO to Na′II–OC matches the respective experimental barrier observed in the NaX zeolite.


 Please wait while we load your content...
Please wait while we load your content...