Complexation of carboxylate on smectite surfaces
Abstract
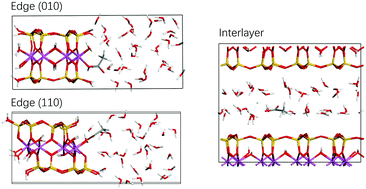
We report a first principles molecular dynamics (FPMD) study of carboxylate complexation on clay surfaces. By taking acetate as a model carboxylate, we investigate its inner-sphere complexes adsorbed on clay edges (including (010) and (110) surfaces) and in interlayer space. Simulations show that acetate forms stable monodentate complexes on edge surfaces and a bidentate complex with Ca2+ in the interlayer region. The free energy calculations indicate that the complexation on edge surfaces is slightly more stable than in interlayer space. By integrating pKas and desorption free energies of Al coordinated water calculated previously (X. Liu, X. Lu, E. J. Meijer, R. Wang and H. Zhou, Geochim. Cosmochim. Acta, 2012, 81, 56–68; X. Liu, J. Cheng, M. Sprik, X. Lu and R. Wang, Geochim. Cosmochim. Acta, 2014, 140, 410–417), the pH dependence of acetate complexation has been revealed. It shows that acetate forms inner-sphere complexes on (110) in a very limited mildly acidic pH range while it can complex on (010) in the whole common pH range. The results presented in this study form a physical basis for understanding the geochemical processes involving clay–organics interactions.


 Please wait while we load your content...
Please wait while we load your content...