High coverage water adsorption on CuO(011) surface†
Abstract
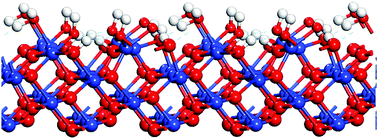
Spin-polarized density functional theory calculations (GGA+U) and atomic thermodynamics have been used to study the adsorption of water on the CuO(011) surface at different coverages. It was found that H2O molecular adsorption on CuO(011) surface is energetically favorable for one H2O molecule, but dissociative adsorption is preferred for two and three molecules, while a mixed molecular and dissociative coadsorption is favorable for four water molecules. The phase diagram of water adsorption on the CuO(011) surface shows that the adsorption of three and four water molecules is favorable thermodynamically. Different single-water adsorption states were analyzed by the Boltzmann model at different temperatures. The adsorption energy is contributed to by the surface uncoordinated copper and oxygen atoms, and by hydrogen chemical bonding. The energetic trends are related to the underlying electronic mechanisms.


 Please wait while we load your content...
Please wait while we load your content...