Mixing scheme of an aqueous solution of tetrabutylphosphonium trifluoroacetate in the water-rich region†
Abstract
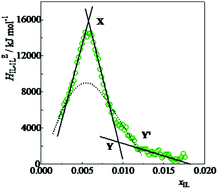
We have studied the mixing scheme of an aqueous solution of ionic liquid tetrabutylphosphonium trifluoroacetate, [P4,4,4,4]CF3COO, in the water-rich region. The mixture shows phase separation with a lower critical solution temperature. To learn how the solute [P4,4,4,4]CF3COO species interact with each other in the dilute region, the third derivative quantities of Gibbs energy in terms of enthalpy, HEIL–IL, and volume, VEIL–IL, are evaluated. In the present study using up to the third derivative quantities, we conclude that [P4,4,4,4]CF3COO turns out to be an extremely strong hydrophobe. This finding may hint that the present IL is not dissociated in this water-rich region, despite the large dielectric permittivity of solvent water and as such it works as an extremely strong hydrophobe. An earlier 1-propanol (1P) probing study showed that [P4,4,4,4]+ is a significant amphiphile with a strong hydrophobicity and an equally strong hydrophilicity, and CF3COO− is a modest amphiphile. However, the 1-propanol (1P) probing methodology effectively uses the fourth derivative of G, and is capable of providing deeper information on the effect of the test solute on H2O. To reconcile the present conclusion with the earlier observations described above, we propose to conduct the 1P-probing of the ionic liquid salt, [P4,4,4,4]CF3COO.


 Please wait while we load your content...
Please wait while we load your content...