A water-mediated and substrate-assisted aminoacylation mechanism in the discriminating aminoacyl-tRNA synthetase GlnRS and non-discriminating GluRS†
Abstract
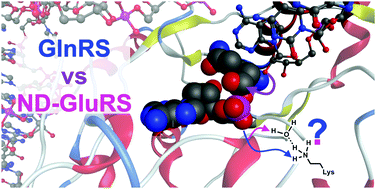
Glutaminyl-tRNA synthetase (GlnRS) catalyzes the aminoacylation of glutamine to the corresponding tRNAGln. However, most bacteria and all archaea lack GlnRS and thus an indirect noncanonical aminoacylation is required. With the assistance of a non-discriminating version of Glutamyl-tRNA synthetases (ND-GluRS) the tRNAGln is misaminoacylated by glutamate. In this study, we have computationally investigated the aminoacylation mechanism in GlnRS and ND-GluRS employing Molecular Dynamics (MD) simulations, Quantum Mechanics (QM) cluster and Quantum Mechanics/Molecular Mechanics (QM/MM) calculations. Our investigations demonstrated the feasibility of a water-mediated, substrate-assisted catalysis pathway with rate limiting steps occurring at energy barriers of 25.0 and 25.4 kcal mol−1 for GlnRS and ND-GluRS, respectively. A conserved lysine residue participates in a second proton transfer to facilitate the departure of the adenosine monophosphate (AMP) group. Thermodynamically stable (−29.9 and −9.3 kcal mol−1 for GlnRS and ND-GluRS) product complexes are obtained only when the AMP group is neutral.


 Please wait while we load your content...
Please wait while we load your content...