Heavy ligand atom induced large magnetic anisotropy in Mn(ii) complexes†
Abstract
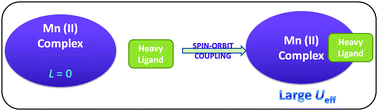
In the search for single molecule magnets, metal ions are considered pivotal towards achieving large magnetic anisotropy barriers. In this context, the influence of ligands with heavy elements, showing large spin–orbit coupling, on magnetic anisotropy barriers was investigated using a series of Mn(II)-based complexes, in which the metal ion did not have any orbital contribution. The mixing of metal and ligand orbitals was achieved by explicitly correlating the metal and ligand valence electrons with CASSCF calculations. The CASSCF wave functions were further used for evaluating spin–orbit coupling and zero-field splitting parameters for these complexes. For Mn(II) complexes with heavy ligand atoms, such as Br and I, several interesting inter-state mixings occur via the spin–orbit operator, which results in large magnetic anisotropy in these Mn(II) complexes.


 Please wait while we load your content...
Please wait while we load your content...