Strong intermolecular antiferromagnetic verdazyl–verdazyl coupling in the solid state†
Abstract
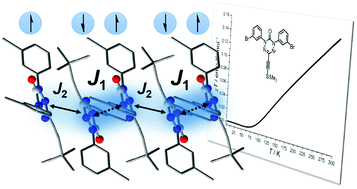
Strong magnetic couplings are generally observed intramolecularly in organic diradicals or in systems in which they are promoted by crystal engineering strategies involving, for example, transition metal ligation. We herein present a strong intermolecularly coupling verdazyl radical in the solid state without the use of such design strategies. The crystal structure of an acetylene-substituted verdazyl radical shows a unique antiparallel face-to-face orientation of the neighboring verdazyl molecules along with verdazyl–acetylene interactions giving rise to an alternating antiferromagnetic Heisenberg chain. Single crystal structural data at 80, 100, 173, and 223 K show that one of the π-stacking distances depends on temperature, while heat capacity data indicate the absence of a phase transition. Based on this structural input, broken symmetry DFT calculations predict a change from an alternating linear Heisenberg chain with two comparable coupling constants J1 and J2 at higher temperatures towards dominant pair interactions at lower temperatures. The predicted antiferromagnetic coupling is confirmed experimentally by magnetic susceptibility, solid-state EPR and NMR spectroscopic results.


 Please wait while we load your content...
Please wait while we load your content...