A novel explanation for the increased conductivity in annealed Al-doped ZnO: an insight into migration of aluminum and displacement of zinc†
Abstract
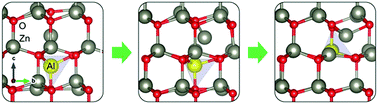
A combined experimental and first-principles study is performed to study the origin of conductivity in ZnO:Al nanoparticles synthesized under controlled conditions via a reflux route using benzylamine as a solvent. The experimental characterization of the samples by Raman, nuclear magnetic resonance (NMR) and conductivity measurements indicates that upon annealing in nitrogen, the Al atoms at interstitial positions migrate to the substitutional positions, creating at the same time Zn interstitials. We provide evidence for the fact that the formed complex of AlZn and Zni corresponds to the origin of the Knight shifted peak (KS) we observe in 27Al NMR. As far as we know, the role of this complex has not been discussed in the literature to date. However, our first-principles calculations show that such a complex is indeed energetically favoured over the isolated Al interstitial positions. In our calculations we also address the charge state of the Al interstitials. Further, Zn interstitials can migrate from AlZn and possibly also form Zn clusters, leading to the observed increased conductivity.


 Please wait while we load your content...
Please wait while we load your content...