Photocatalytic hydrogenation of furan to tetrahydrofuran in alcoholic suspensions of metal-loaded titanium(iv) oxide without addition of hydrogen gas†
Abstract
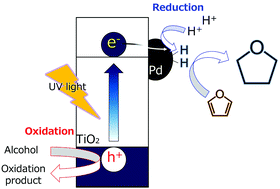
The use of metal co-catalysts broadens the application of photocatalytic reduction without the use of dihydrogen (H2) gas. We examined photocatalytic hydrogenation of furan, a representative heterocyclic compound and a compound derived from biomass, in alcoholic suspensions of metal-loaded titanium(IV) oxide (TiO2) under a H2-free condition and we found that furan was almost quantitatively hydrogenated to tetrahydrofuran with a high apparent quantum efficiency of 37% at 360 nm when palladium was used as a co-catalyst. Effects of different metal co-catalysts, different amounts of the co-catalyst, the type of TiO2, the type of alcohol, light wavelength and reusability for furan hydrogenation were investigated. Based on the results, the functions of TiO2 and the co-catalyst and the reaction process are discussed.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...