On the feasibility of reactions through the fullerene wall: a theoretical study of NHx@C60†
Abstract
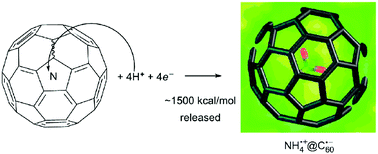
We propose a new approach to the synthesis of AHx@fullerene structures via reactions through the fullerene wall. To investigate the feasibility of the approach, the step-by-step hydrogenation of the template endofullerene N@C60 up to NH4@C60 has been studied using DFT and MP2 calculations. Protonation of the endohedral guest through the fullerene wall is competitive with escape of the guest, whereas reaction with a hydrogen atom is less favorable. Each protonation step is highly exothermic, so that less active acids can also protonate the guest with less accumulation of energy. The final product, NH4@C60 is a novel concentric ion pair NH4+@C60˙− in which the charge-centers of the two ions coincide.


 Please wait while we load your content...
Please wait while we load your content...