The first water coordination sphere of lanthanide(iii) motexafins (Ln-Motex2+, Ln = La, Gd, Lu) and its effects on structures, reduction potentials and UV-vis absorption spectra. Theoretical studies†
Abstract
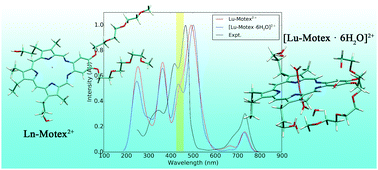
Density functional calculations have been performed to study selected hydrated lanthanide(III) motexafins (Ln-Motex2+, Ln = La, Gd, Lu) by using energy-consistent 4f-in-core lanthanide pseudopotentials to include the major relativistic effects due to the heavy metals. The maximum number (n) of water molecules bound strongly to [Ln-Motex]2+ (Ln = La, Gd, Lu) was determined to be 6 by calculating the change of the Gibbs energies for the reactions [Ln-Motex(H2O)n]2+ + H2O → [Ln-Motex(H2O)n+1]2+. The number of water molecules coordinated directly to Ln3+ was found to be 3 for La, and 2 for Gd and Lu. The explicit treatment of the tightly bound water molecules in [Ln-Motex(H2O)6]2+ in combination with the COSMO solvation model yielded calculated reduction potentials and UV-vis absorption spectra in good agreement with available experimental data.


 Please wait while we load your content...
Please wait while we load your content...