Catalysis by solvation rather than the desolvation effect: exploring the catalytic efficiency of SAM-dependent chlorinase†
Abstract
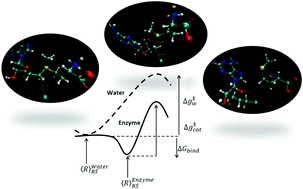
Chlorinase SalL halogenate S-adenosyl-L-methionine (SAM) reacts with chloride to generate 5′-chloro-5′-deoxyadenosine and L-methionine through a nucleophilic substitution mechanism. Although it is known that chlorinase enhances the rate of reaction by a factor of 1.2 × 1017 fold, it is not entirely clear how this is accomplished. The search for the origin of the catalysis of chlorinase and other enzymes has led to a desolvation hypothesis. In the present work, we have used well defined computational simulations in order to evaluate the origin of the catalytic efficiency of chlorinase. The results demonstrate that the catalytic effect of chlorinase is associated with the fact that Cl− is “solvated” by the protein more than by the reference solution reaction, which is not in accordance with proposed catalysis by desolvation. It is found that chlorinase SalL active sites provide electrostatic stabilization of the transition state which is the origin of its catalytic effect.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...