Exploring the chemical kinetics of partially oxidized intermediates by combining experiments, theory, and kinetic modeling
Abstract
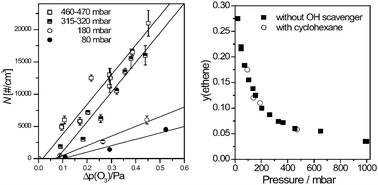
Partially oxidized intermediates play a central role in combustion and atmospheric chemistry. In this perspective, we focus on the chemical kinetics of alkoxy radicals, peroxy radicals, and Criegee intermediates, which are key species in both combustion and atmospheric environments. These reactive intermediates feature a broad spectrum of chemical diversity. Their reactivity is central to our understanding of how volatile organic compounds are degraded in the atmosphere and converted into secondary organic aerosol. Moreover, they sensitively determine ignition timing in internal combustion engines. The intention of this perspective article is to provide the reader with information about the general mechanisms of reactions initiated by addition of atomic and molecular oxygen to alkyl radicals and ozone to alkenes. We will focus on critical branching points in the subsequent reaction mechanisms and discuss them from a consistent point of view. As a first example of our integrated approach, we will show how experiment, theory, and kinetic modeling have been successfully combined in the first infrared detection of Criegee intermediates during the gas phase ozonolysis. As a second example, we will examine the ignition timing of n-heptane/air mixtures at low and intermediate temperatures. Here, we present a reduced, fuel size independent kinetic model of the complex chemistry initiated by peroxy radicals that has been successfully applied to simulate standard n-heptane combustion experiments.
- This article is part of the themed collections: PCCP Perspectives and 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...