Laser photolysis studies of ω-bond dissociation in aromatic carbonyls with a C–C triple bond stimulated by triplet sensitization†
Abstract
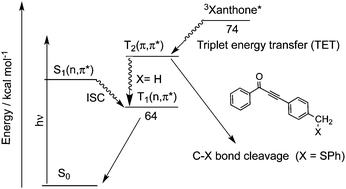
We have prepared three types of carbonyl compounds, benzoylethynylmethyl phenyl sulfide (2@SPh), (p-benzoyl)phenylethynylmethyl phenyl sulfide (3@SPh) and p-(benzoylethynyl)benzyl phenyl sulfide (4@SPh) with benzoyl and phenylthiylmethyl groups, which are interconnected with a C–C triple bond and a phenyl ring. Laser flash photolysis of 3@SPh and 4@SPh in acetonitrile provided the transient absorption spectra of the corresponding triplet states where no chemical reactions were recognized. Upon laser flash photolysis of 2@SPh, the absorption band due to the phenylthiyl radical (PTR) was obtained, indicating that the C–S bond cleaved in the excited state. Triplet sensitization of these carbonyl compounds using acetone and xanthone was conducted using laser photolysis techniques. The formation of triplet 3@SPh was seen in the transient absorption, whereas the PTR formation was observed for 2@SPh and 4@SPh, indicating that the triplet states were reactive for the C–S bond dissociation. The C–S bond dissociation mechanism for 4@SPh upon triplet sensitization is discussed in comparison with those for 2@SPh and 3@SPh.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...