How predictive could alchemical derivatives be?
Abstract
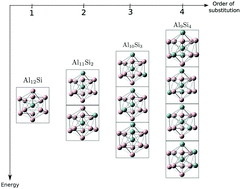
The chemical space contains all possible compounds that can be imagined. Its size easily equals the number of fundamental particles in the observable universe. Rational design of compounds aims to find those sectors of the chemical space where compounds optimize a set of desired properties. Then, rational design demands tools to efficiently navigate the chemical space. Ab initio alchemical derivatives offer the possibility to navigate, without empiricism, the energy landscape through alchemical transformations. An alchemical transformation is any process, physical or fictitious, that connects to points in the chemical space. In this work, those transformations are constructed as a perturbative expansion of the energy with respect to perturbations in the stoichiometry. The response functions of that expansion are what is called alchemical derivatives. In this work we assess how effective alchemical derivatives are in predicting energy changes associated to changes in the composition. We do this by including in the expansion, for the first time, electrostatic, polarization and electron-transfer effects. The system we chose is one that challenges alchemical derivatives because none of these effects dominates its behavior. The transmutations studied here correspond to substitutional doping of Al13 with up to four atoms of Si, Al13−nSin. Two types of transformations are considered, those in which the number of electrons remains constant and those in which the number of electrons also changes. It is found that contrary to what has been reported before, polarization cannot be neglected. If polarization is not included, alchemical derivatives fail to predict the change of energy and the relative energy between isomers. For isoelectronic substitution of four or more atoms, the perturbative approach collapses because the strength of the perturbation becomes too strong to guarantee convergence of the series. It is shown, however, that if only one atom is mutated at a time, alchemical derivatives rank pretty well the isomers of Al13−nSin according to their energy. In the case of non-isoelectronic transformations, it is observed that the series rapidly diverges with increasing number of electrons. In this situation, it becomes more important to keep the degree of transmutation of the parent system small.


 Please wait while we load your content...
Please wait while we load your content...