Effect of lipid shape on toroidal pore formation and peptide orientation in lipid bilayers
Abstract
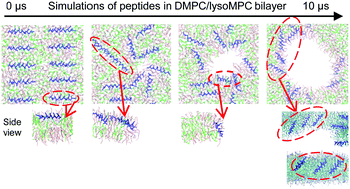
Amphiphilic peptides of different lengths were simulated with lipid bilayers composed of 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) and 1-myristoyl-2-hydroxy-sn-glycero-3-phosphocholine (lysoMPC) in different ratios. Simulations of lipid bilayers without peptides show that the bilayers with more lysoMPC become more disordered and thinner. Amphiphilic peptides added to this simulation do not insert into the DMPC bilayer at a low peptide/lipid ratio (P/L ≤ 1/50), while they do insert into the DMPC/lysoMPC bilayer and form a toroidal pore even at such a low P/L ratio, where the pore edge is surrounded by lysoMPC rather than by DMPC. In particular, upon pore formation, peptides migrate toward the edge of a pore and become tilted, showing transmembrane alignment regardless of the peptide length, in qualitative agreement with experiments. This pore formation occurs more frequently in larger bilayers that allow greater curvature, indicating that bilayer curvature is important for pore formation. These results indicate that the addition of lysoMPC induces a thinner bilayer with greater curvature, and thus the bilayer with lysoMPC can be more easily penetrated by peptides, leading to the formation of a toroidal pore stabilized by peptides and lysoMPC. These findings help explain experimental observations of the effect of the inverted cone-shaped lyso-lipid on pore formation and peptide orientation, and also support the experimental suggestion regarding the formation of an iris-like ring of helices lining a toroidal pore.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...