PPh4Cl in aqueous solution – the aggregation behavior of an antagonistic salt
Abstract
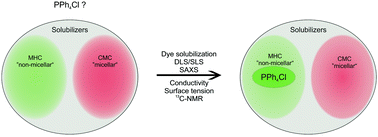
The initial definition of hydrotropy by Neuberg in 1916 describes a hydrotrope as a molecule which enhances the solubilization of hydrophobic substances in water. Sodium dodecyl sulfate (SDS) and sodium xylene sulfonate (SXS) are typical representatives fulfilling this old definition. They are either surfactants with a critical micellar concentration (CMC) or hydrotropes in the current sense of the term, showing a minimum hydrotrope concentration (MHC), respectively. In the present contribution, we consider the antagonistic salt PPh4Cl as a hydrotrope. Surface tension measurements and solubilization experiments on a hydrophobic dye confirm the solubilization behavior of PPh4Cl, which is in-between the one of SDS and SXS. With the help of scattering techniques (DLS, SLS, SAXS), NMR and conductivity measurements, we show that in contrast to SDS as a hydrotrope with an inherent CMC, PPh4Cl does not exhibit mesoscale aggregation. Therefore, PPh4Cl can be classified rather as a hydrotrope in the modern sense, with an inherent MHC just as SXS.


 Please wait while we load your content...
Please wait while we load your content...