Polysaccharide zeta-potentials and protein-affinity†
Abstract
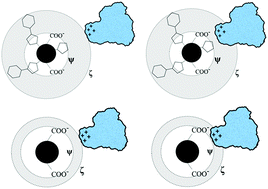
The ζ-potential, a parameter typically obtained by model-dependent transformation of the measured electrophoretic mobility, is frequently used to understand polysaccharide–protein complexation. We tested the hypothesis that two anionic polysaccharides with identical ζ-potentials would show equal binding affinity to the protein β-lactoglobulin (BLG). We selected two polysaccharide polyelectrolytes (PE) with very different structures: hyaluronic acid (HA) and tragacanthin (TG). Highly precise (±0.1%) turbidimetric titrations were performed to determine critical pH values of complex formation; and PE ζ-potentials were measured for different ionic strengths I at those critical pH values. While phase boundaries (pHcvs. I) showed that HA binds to BLG more strongly (e.g. at a lower pH, for fixed I), comparisons made at fixed ζ-potential indicated that TG binds more strongly. The source of this contradiction is the effect of the bulky side chains of TG on its friction coefficient which diminishes its mobility and hence the resultant ζ-potential; while having a distinctly separate effect on the interaction between BLG and the carboxylated backbone of TG. Thus, unless the locus of the bound protein coincides with the shear plane, the ζ-potential does not directly contribute to the electrostatic PE–protein interaction.


 Please wait while we load your content...
Please wait while we load your content...