Gas-phase vibrational spectroscopy of triphenylamine: the effect of charge on structure and spectra†
Abstract
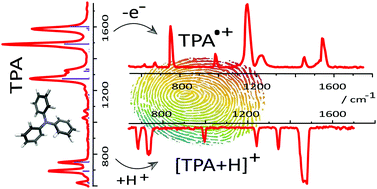
The effect of ionization by oxidation and protonation on the structure and IR spectrum of isolated, gas-phase triphenylamine (TPA) has been investigated by infrared multiple photon dissociation (IRMPD) spectroscopy in the fingerprint range from 600 cm−1 to 1800 cm−1 using an infrared free electron laser. IR spectra calculated using density functional theory (DFT) convincingly reproduce the experimental data. Spectral and structural differences are identified among neutral TPA, TPA˙+ and protonated TPA and qualitatively related to effects of resonance delocalization. As a consequence of electron delocalization, computed structural parameters for TPA remain virtually unchanged upon removal of an electron. Nonetheless, CC and CN stretching vibrations in the IR spectra of TPA˙+ undergo a red shift of up to 52 cm−1 as compared to those in TPA. Since ionization also strongly influences the relative band intensities, a vibrational projection analysis was used to correlate vibrational modes of TPA with those of TPA˙+. The experimental IR spectrum of gas-phase protonated TPA indicates that protonation occurs on the nitrogen atom, despite delocalization of the lone electron pair. Upon protonation, the structure changes from the nearly planar geometry to a near-tetrahedral configuration.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...