Structural changes and picosecond to second dynamics of cytochrome c in interaction with nitric oxide in ferrous and ferric redox states†‡
Abstract
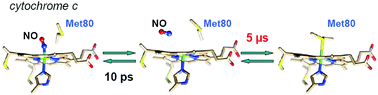
Apart from its role in electron transfer, mitochondrial cytochrome c also plays a role in apoptosis and is subject to nitrosylation. The cleavage of the Fe–Met80 bond plays a role in several processes including the release of Cyt c from mitochondria or increase of its peroxidase activity. Nitrosylation of Cyt c precludes the reformation of the disrupted Fe–Met80 bond and was shown to occur during apoptosis. These physiological properties are associated with a conformational change of the heme center of Cyt c. Here, we demonstrate that NO binding induces pronounced heme conformational changes in the six-coordinate Cyt c–NO complex. Equilibrium and time-resolved Raman data reveal that the heme structural conformation depends both on the nature of the distal iron ligand (NO or Met80) and on the Fe2+ or Fe3+ heme redox state. Upon nitrosylation, the heme ruffling distortion is greatly enhanced for ferrous Cyt c. Contrastingly, the initial strong heme distortion in native ferric Cyt c almost disappears after NO binding. We measured the heme coordination dynamics in the picosecond to second time range and identified Met80 and NO rebinding phases using time-resolved Raman and absorption spectroscopies. Dissociation of NO instantly produces 5-coordinate heme with a domed structure which continues to rearrange within 15 ps, while the initial ruffling distortion disappears. The rates of Cyt c–NO complex formation measured by transient absorption are kon = 1.81 × 106 M−1 s−1 for ferric Cyt c and 83 M−1 s−1 for ferrous Cyt c. After NO dissociation and exit from the heme pocket, the rebinding of Met80 to the heme iron takes place 6 orders of magnitude more slowly (3–5 μs) than Met80 rebinding in the absence of NO (5 ps). Altogether, these data reveal the structural and dynamic properties of Cyt c in interaction with nitric oxide relevant for the molecular mechanism of apoptosis.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...