Self-assembled nanocapsules in water: a molecular mechanistic study
Abstract
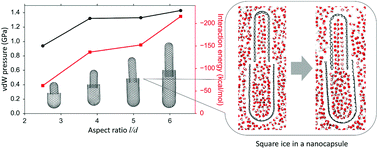
The self-assembly mechanism of one-end-open carbon nanotubes (CNTs) suspended in an aqueous solution was studied by molecular dynamics simulations. It was shown that two one-end-open CNTs with different diameters can coaxially self-assemble into a nanocapsule. The nanocapsules formed were stable in aqueous solution under ambient conditions, and the pressure inside the nanocapsule was much higher than the ambient pressure due to the van der Waals interactions between the two parts of the nanocapsule. The effects of the normalized radius difference, normalized inter-tube distance and aspect ratio of the CNT pairs were systematically explored. The electric field response of the nanocapsules was studied using ab initio molecular dynamics simulations, which shows that the nanocapsules can be opened by applying an external electric field, due to the polarization of carbon atoms. This discovery not only sheds light on a simple yet robust nanocapsule self-assembly mechanism, but also underpins potential innovations in drug delivery, nano-reactors, etc.


 Please wait while we load your content...
Please wait while we load your content...