CoN3 embedded graphene, a potential catalyst for the oxygen reduction reaction from a theoretical perspective†
Abstract
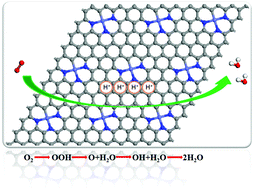
Non-precious metal catalysts have attracted particular interest in recent years due to their promising ORR (oxygen reduction reaction) activity in fuel cells. In this work, the structural stability and ORR mechanism of CoN3 embedded graphene have been studied theoretically in acid media. The results indicate that CoN3 embedded graphene is stable thermodynamically. The kinetically most favorable reaction pathway for the ORR is a four-electron process. The process of OOH hydrogenation to generate O + H2O is the most favorable pathway. In the rate determining step, the energy barrier is 0.38 eV, much smaller than the theoretical value of ∼0.80 eV for pure Pt. The predicted working potential is 0.4 V for the most favorite pathway. Besides the lower energy barrier, the smaller Tafel slope compared with pure Pt in both low and high overpotential regions also suggests that CoN3 embedded graphene is a promising electrocatalyst for the ORR.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...