How active sites facilitate charge-transfer interactions of silver and gold clusters with TCNQ?†
Abstract
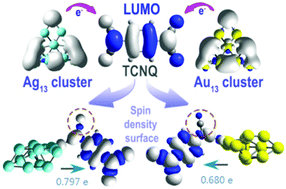
Utilizing a strong electron acceptor molecule tetracyanoquinodimethane (TCNQ) as probe, we demonstrate how the electronic features and geometric sites determine charge-transfer interactions of noble metal clusters with organic molecules. First-principle calculations by searching local minimum energies suggest that the TCNQ complexes with Ag13, Au12Ag1, Au1Ag12 and Au13 all favor edge-site adsorption, and their structures are highly comparable and possess metal–N–metal bonds. Further analysis on frontier molecular orbitals (FMOs) and natural population analysis (NPA) reveals that it's easier for Ag13/Au1Ag12 clusters to transfer electrons to TCNQ as compared with Au13/Ag1Au12. Spin density isosurfaces indicate that the charge transfer from these 13-atom clusters to TCNQ leads to electronic shell closure of the metal clusters. The difference in the electronegativities of Ag and Au, as well as the significant relativistic effect of gold, results in varying donor–accepter interactions sensitive to the coordination number of the doping atom for both Au12Ag1 and Au1Ag12 clusters.


 Please wait while we load your content...
Please wait while we load your content...