First-principles study of adsorption–desorption kinetics of aqueous V2+/V3+ redox species on graphite in a vanadium redox flow battery†
Abstract
Vanadium redox flow batteries (VRFBs) represent a promising solution to grid-scale energy storage, and understanding the reactivity of electrode materials is crucial for improving the power density of VRFBs. However, atomistic details about the interactions between vanadium ions and electrode surfaces in aqueous electrolytes are still lacking. Here, we examine the reactivity of the basal (0001) and edge (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) graphite facets with water and aqueous V2+/V3+ redox species at 300 K employing Car–Parrinello molecular dynamics (CPMD) coupled with metadynamics simulations. The results suggest that the edge surface is characterized by the formation of ketonic C
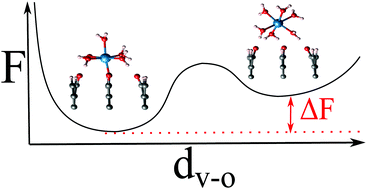
0) graphite facets with water and aqueous V2+/V3+ redox species at 300 K employing Car–Parrinello molecular dynamics (CPMD) coupled with metadynamics simulations. The results suggest that the edge surface is characterized by the formation of ketonic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups due to complete water dissociation into the H/O/H configuration with surface O atoms serving as active sites for adsorption of V2+/V3+ species. The formation of V–O bonds at the surface should significantly improve the kinetics of electron transfer at the edge sites, which is not the case for the basal surface, in agreement with the experimentally hypothesized mechanism.
O functional groups due to complete water dissociation into the H/O/H configuration with surface O atoms serving as active sites for adsorption of V2+/V3+ species. The formation of V–O bonds at the surface should significantly improve the kinetics of electron transfer at the edge sites, which is not the case for the basal surface, in agreement with the experimentally hypothesized mechanism.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...